Assessing Functionality of GFP+ Hematopoietic Stem Cells in Neonate Bone Marrow
Engineering
BME 195
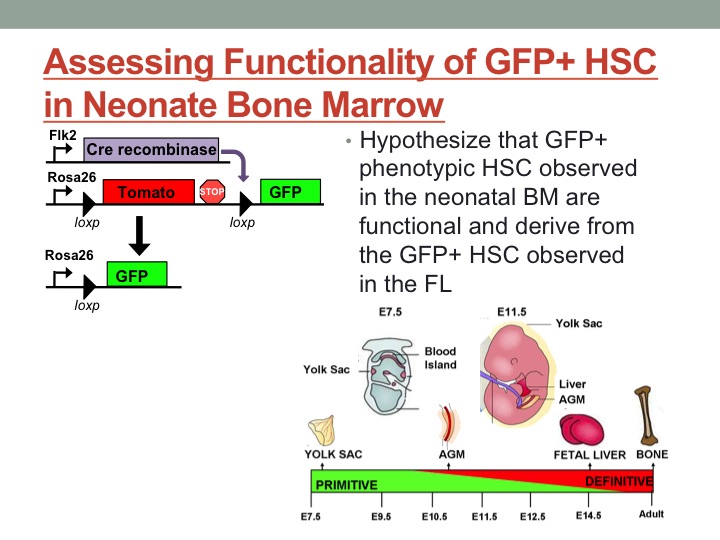
Hematopoietic stem cells (HSC) are the most well-characterized adult stem cell population; it is well established that a single stem cell can reconstitute the entire blood system of an irradiated adult recipient, and basic differentiation pathways between a stem cell and all mature cell lineages have been reasonably-well characterized. Hematopoietic stem cells are also currently the only stem cell population routinely used for transplantation in a clinical setting. Despite several decades of research, however, the mechanisms regulating the establishment of adult HSC during fetal development remain obscure. Understanding the developmental pathways leading to establishment of adult HSC as well as determining the mechanisms that regulate long-term self-renewal capability are critical to furthering the success rate of cell-based treatment, including the elusive goal of generating adult HSC from pluripotent stem cells. To clarify differentiation pathways in the adult hematopoietic system our lab recently developed a lineage tracing mouse model that maps cells with a history of expression of the tyrosine kinase receptor Flk2. In this model, cells that pass through a Flk2+ stage will switch expression of a dual color reporter from Tom+, a red fluorescing protein, to GFP, green fluorescent protein, expression. This switch is irreversible; a GFP+ cell cannot give rise to a Tom+ cell. Functional and phenotypic characterization of this model revealed that all adult HSC are Tom+, indicating that they cannot have derived from a GFP+ cell with a history of Flk2 expression. Recently, characterization of fetal development in the Flk2 lineage tracing mouse model revealed the presence of a novel GFP+ HSC population. This novel GFP+ population functions as an adult HSC upon transplantation, as it is capable of supporting long-term reconstitution of all mature lineages upon transplantation into irradiated adult recipients. Additionally, these GFP+ HSC are functionally distinct from their Tom+ counterparts in that they exhibit differential lineage bias and generate distinct subsets of immune cells. However, as we have demonstrated conclusively that all adult HSC are Tom+, it is impossible for these GFP+ HSC to contribute to the adult HSC population in situ. Thus, this GFP+ HSC represents a functionally-distinct, developmentally-regulated hematopoietic wave. A developmentally-restricted wave of adult-like HSC has never been described before, and represents a unique opportunity to investigate the mechanisms that regulate long-term persistence and self-renewal capability of HSC in vivo. My project focuses on understanding the mechanisms limiting GFP+ HSC to a developmentally-restricted window. One possible explanation for the absence of GFP+ HSC in the adult bone marrow is that these developmentally-restricted HSC lack the capacity to home to the bone marrow, and therefore never take up residence within the adult HSC niche. Alternatively, these HSC might be capable of homing to the bone marrow but therein lack the capacity to self-renew within the bone marrow niche. My phenotypic analysis revealed the presence of GFP+ HSCs in the neonate bone marrow; however, it is unclear whether these phenotypic HSC are functional. To address this question, I functionally analyzed Tom+ and GFP+ stem cells by performing transplantation assays. I isolated purified Tom+ and GFP+ stem cell fractions by flow cytometry and assessed self-renewal and differentiation by transplanting into sub-lethally irradiated wildtype hosts. I measured the contribution of these cell populations to reconstitution of mature blood cells in the peripheral blood (PB) over four months. Preliminary data indicate that the GFP+ fraction contribute to PB reconstitution at early timepoints, but not in the long-term. These data indicate that the GFP+ cells do not represent functional HSC, but instead should be designated as non-self-renewing progenitor cells. Additional experiments are in progress to replicate this finding. The results from this project will help us to understand the hematopoietic waves during development and the mechanism that control the lifespan of a hematopoietic stem cell in vivo.