Beyond cyclosporine A: conformation-dependent passive membrane permeabilities of cyclic peptide natural products
Physical and Biological Sciences
Independent Study
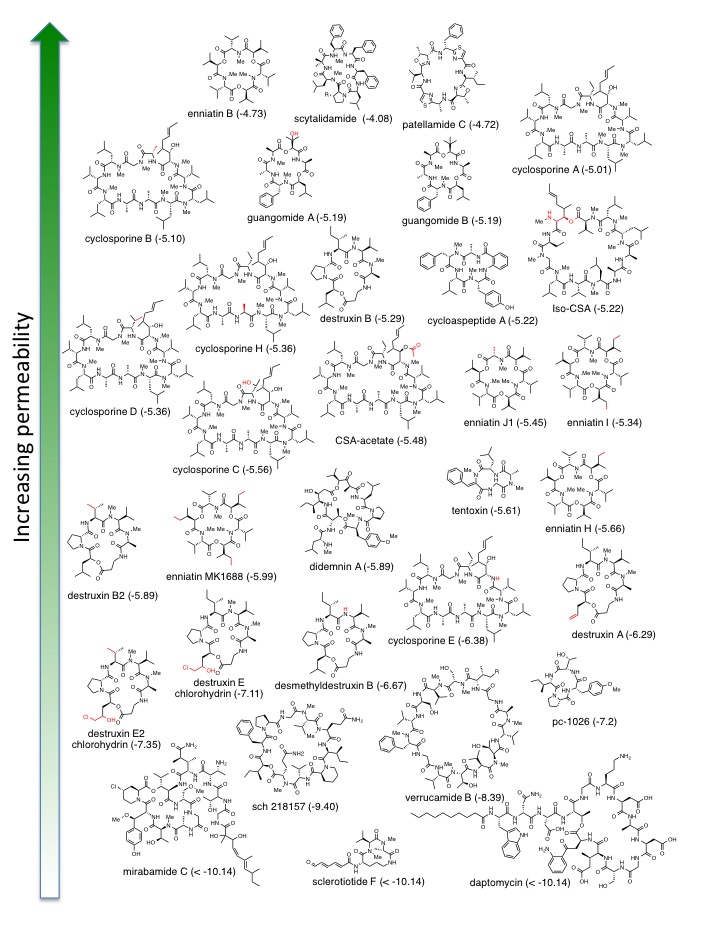
In drug development, one of the most important characteristics is the ability of a molecule to cross a biological cell membrane. If it is unable to successfully do so, the drug cannot be marketed as an oral agent, despite its possible potency or promise. This restriction severely inhibits the availability of a pharmaceutical that may have incredible biological activity and extraordinary benefit for human health. Cyclic peptides are a class of molecules that are much larger (at the molecular level) than conventional small molecule drugs. This property has been typically thought to prevent them from crossing the human intestinal cell membrane, and cyclic peptides have been subsequently pushed aside in drug discovery interest despite their unique ability and potential to inhibit biological targets that small molecules cannot access. Here we present the passive membrane permeabilities of 39 cyclic peptide natural products, and interpret the results using a computational permeability prediction algorithm based on their known or calculated three-dimensional conformations. We found that the permeabilities of these compounds, measured in a parallel artificial membrane permeability assay (PAMPA), spanned a wide range, with the expected result that the least permeable compounds were among the largest and/or had multiple charged or highly polar residues. However, even among the highly lipophilic compounds there were large differences in permeability, which could be predicted based on the integration of structural information with our computational algorithm. The identification of common chemical and structural properties among natural product cyclic peptides with passively permeability facilitates the rational design of synthetic or semi-synthetic cyclic peptide drugs.