Single-cell Manipulation Using Nanopipettes
Engineering
BME 195
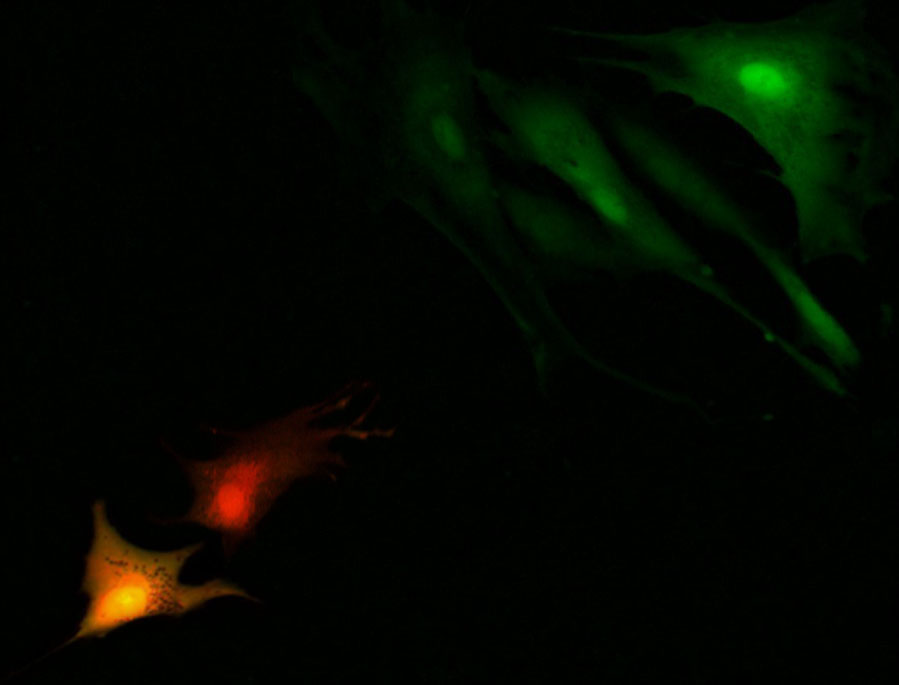
Manipulation and analysis of individual cells is key in understanding processes that control single-cell behavior in a complex environment. Nanotechnology-based tools having high sensitivity and low invasiveness are holding great promises as new biomedical devices for single cell manipulation. The fully electrical read-out as well as the ease and low cost of fabrication are unique features that give nanopipette technology enormous potential. Our group has used nanopipettes as a biosensor, and is now continuing to use nanopipettes for single-cell manipulation. We developed a single-cell manipulation platform based on quartz nanopipettes. The system uses scanning microscopy techniques to position the nanopipette with nanoscale precision. The nanopipette is fitted with electrodes to mediate voltage-dependent injection or aspiration from individual cells. Nanopipettes improve current injection methods due to its high controllability and high viability of cells post injection. Nanopipette tips cause less disruption to the cell membrane and allows injection into single-cells while in their normal plating conditions which improves viability. This technology also allows for multi-component injections. We have shown successful injections into mammalian cells, a technique that is a historically difficult task when using a micropipette. Another needle-based injection method which uses Atomic Force Microscopy (AFM) based techniques have also been demonstrated for injection into mammalian cells but they are limited in terms of throughput and control of injection volumes. Using a similar feedback mechanism as our single-cell injections, our group has continued to use nanopipettes for single-cell manipulations for a project called Single Cell Biopsy, which allows for precise aspiration of contents from a single-cell. We present preliminary results showing the aspiration of minute amounts of cytoplasmic material from a single cell. To further optimize the single-cell manipulation, the devices are interfaced with a microfabricated fluidic chip to immobilize cells onto a 6x6 array, the Cell Sifter. Our technology forms the basis for a fully automated system for high-throughput immobilization and precise injection and aspiration of single cells.