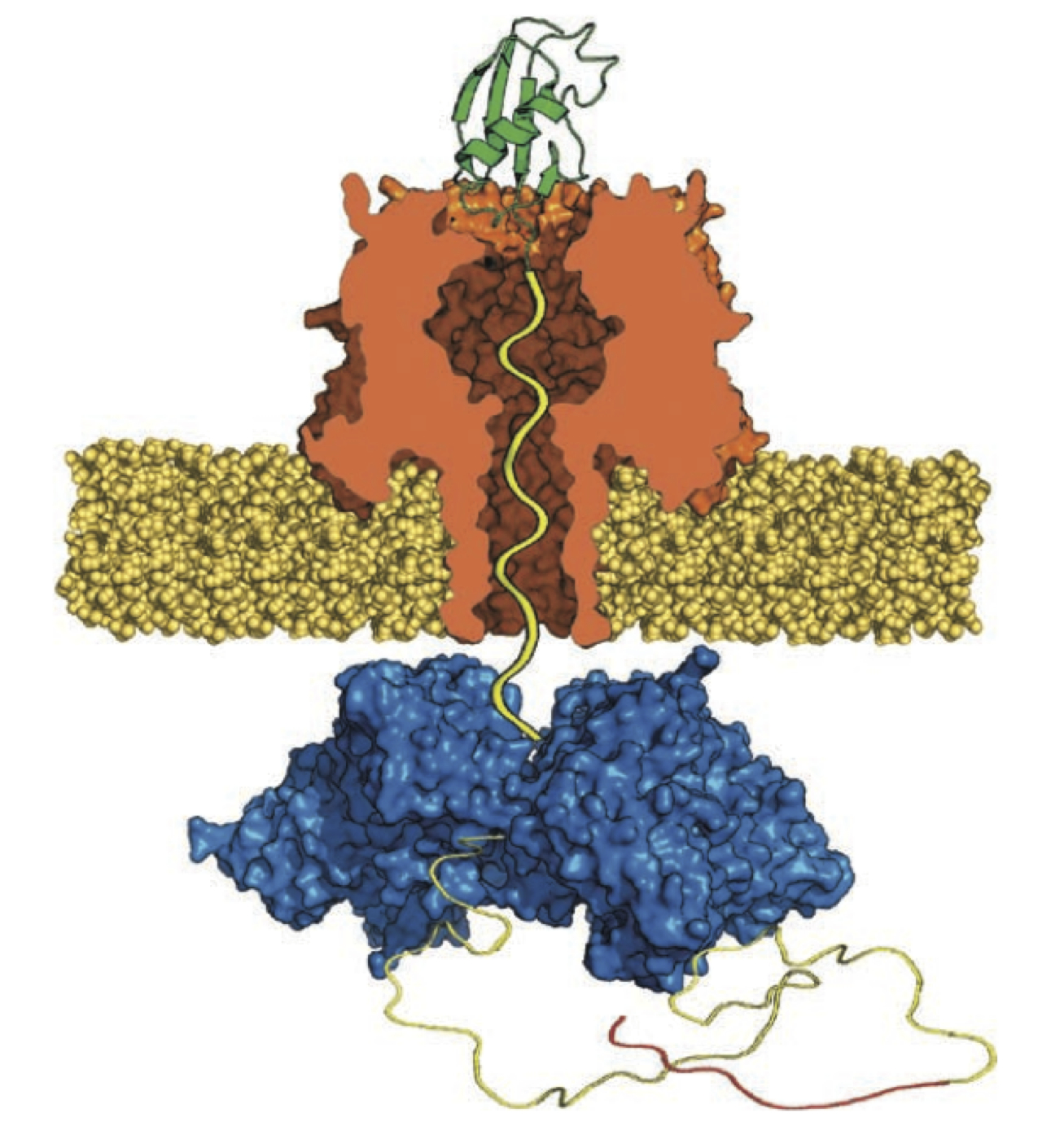
Unfoldase-mediated protein translocation through an α-hemolysin nanopore
Engineering
BME 195, Bioengineering Senior Thesis Research
Nano-scale protein structures, called nanopores, have been adapted for use as biomolecule sensors. A nanopore device allows a single molecule at a time to be analyzed as they pass through the nanometer-sized opening of the protein. Recent advances using enzymes, proteins that perform mechanical work, to control the movement of DNA through a nanopore have set the stage for the production of a nanopore DNA sequencing device. Nanopore sequencing of proteins has also been envisioned. Although proteins have been shown to pass through nanopores, a technique to control the movement of proteins has yet to be demonstrated. Because proteins fold into complex structures that prevent them from being analyzed with a nanopore, a suitable enzyme and strategy must be utilized. Here we describe controlled unfolding and translocation of proteins through the α-hemolysin pore using the enzyme ClpX. Features of individual engineered proteins were detected during translocation. These results demonstrate that enzymes can reproducibly drive proteins through a model nanopore—a feature required for protein sequence analysis using this single-molecule technology.