A vibrational study of Fe2P2O7 and Cr2P2O7 under high-pressures: Structural implications for novel phosphate materials
Physical and Biological Sciences
UCSC Stem Diversity/UC LEADS
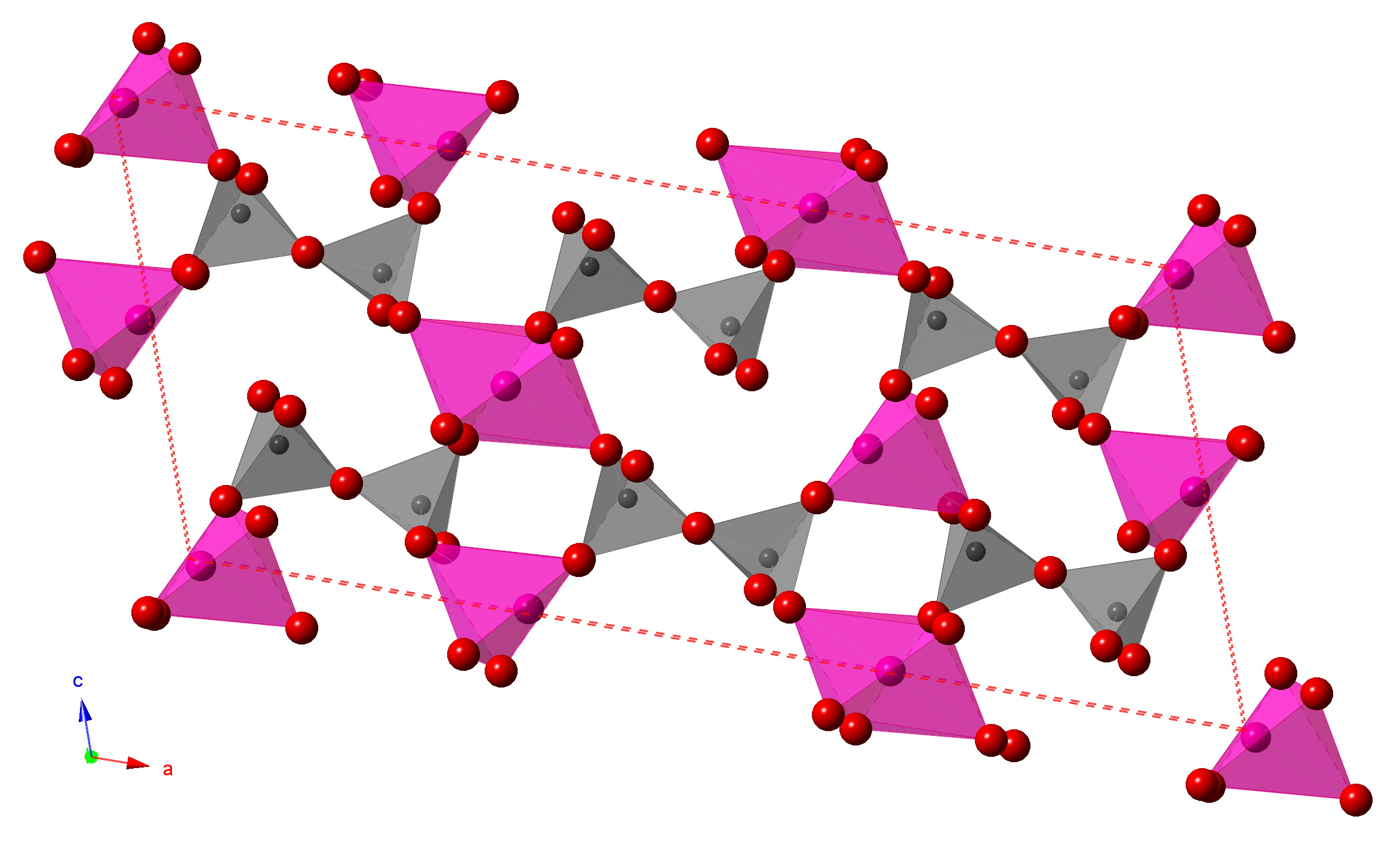
The vibrational properties of synthetic iron diphosphate (Fe2P2O7) and chromium diphosphate (Cr2P2O7) are studied under high-pressure conditions to ~35 and ~22 GPa, respectively, using in situ Raman and infrared spectroscopy. We examine each compound’s structural response to pressure, characterize pressure-induced phase transitions occurring within the compounds and probe their reversibility during decompression. In particular, the response of the initially linear P2O7 dimer group to compression was studied to evaluate the stability of this phosphate configuration under high pressures. The chromium-bearing sample shows normal pressure-induced increases in the frequencies of its infrared modes up to the highest investigated pressures, with coalescence of bands occurring near 6 and 17 GPa: these may be associated with increases in the local symmetry of the P2O7 group. In contrast, the iron sample undergoes a major first order phase transition near ~9 GPa, and an additional possible phase transition near 5.5 GPa. In the latter transition, the Raman-active symmetric stretching vibration of the P-O-P group splits, implying that the symmetry of this group has shifted. At 9 GPa, the initially single strong symmetric PO4 stretching mode splits into four separate modes, and the sole asymmetric PO4 stretching mode splits into two distinct modes. Each of these changes indicate the presence of multiple tetrahedral environments within a new, larger volume unit cell, and the relative frequencies of the split vibrations are consistent with at least one of the P2O7 environments having a markedly narrowed P-O-P angle. Decompression data on the iron-rich compound show that the higher-pressure phase persists to pressures below 2 GPa, but is unquenchable to ambient conditions, with most of the P2O7 groups returning to the initial phase on decompression to ambient conditions. The rationale for the difference between the behaviors of the iron and chromium compounds is likely associated with the compaction of the smaller iron ion within Fe2P2O7 requiring a discontinuous decrease in the P-O-P angle at lower pressures than in the analogous chromium compound. Our results demonstrate that the dimerized P2O7 group remains notably stable under compression to over 20-30 GPa at 300 K, although the P-O-P angle within the iron-rich material undergoes discontinuous decreases under pressure.